 Medinol this week announced it received FDA approval for its Elunir-Perl drug-eluting stent (DES) for the treatment of coronary artery disease.
Medinol this week announced it received FDA approval for its Elunir-Perl drug-eluting stent (DES) for the treatment of coronary artery disease.
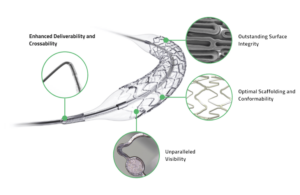
Elunir-Perl is the latest evolution in the company’s Elunir DES product portfolio. It has four radiopaque markers, two at each end of the stent, as well as a hybrid polymer-metal radiopaque catheter tip. The features allow for outstanding visualization during PCI procedures, according to Medinol.
“We are pleased to bring technologies to the U.S. that focus both on benefit to patients as well as unique and meaningful advantages to surgeons, allowing for precise stent placement, shorter procedure times and reduced radiation exposure,” CEO Yoram Richter said in a news release.
The DES will be exclusively available in the U.S. through CoSo Health, a healthcare supply and logistics company.
“We are proud to distribute one of the world’s best drug-eluting stents via our intuitive and easy-to-use SaaS+ platform, which reduces layers in the supply chain,” CoSo Health CEO Jae Lee said.

