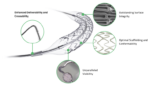
Medinol this week announced it received FDA approval for its Elunir-Perl drug-eluting stent (DES) for the treatment of coronary artery disease. Elunir-Perl is the latest evolution in the company’s Elunir DES product portfolio. It has four radiopaque markers, two at each end of the stent, as well as a hybrid polymer-metal radiopaque catheter tip. The […]
medinol
Medinol stent performs just as well as Medtronic Resolute, study says
The EluNIR drug-eluting stent made by Medinol performed just as well as the Resolute Integrity or Resolute Onyx stents from Medtronic (NYSE:MDT), according to new clinical study results from an international team of researchers. The randomized clinical trial — conducted by researchers from the U.S., Israel, Canada and the Netherlands — involved 2,221 people and compared […]
Cordis, Medinol tout first commercial implants of Elunir drug-eluting stent in U.S.
Cordis and Medinol announced today that the first commercial uses of the Elunir drug-eluting stent in the U.S. were performed at New York-Presbyterian Hospital/Columbia University Medical Center and the Piedmont Heart Institute in Atlanta. The Elunir stent, developed by Medinol and distributed by Cordis, features a metallic spring tip and the narrowest strut width of […]
Cordis, Medinol win FDA nod for Elunir drug-eluting stent
Cordis and Medinol announced today that the FDA approved its Elunir drug-eluting stent for the treatment of patients with narrowing or blocked coronary arteries. The stent, designed by Medinol and distributed exclusively by Cordis in the U.S., features a novel metallic spring tip and the narrowest strut width of any stent on the market in […]
Cordis inks exclusive distribution deal for Medinol’s coronary stent portfolio in US
Cordis has inked an exclusive distribution deal to sell Medinol‘s coronary stents in the U.S., the company reported, adding a cobalt-chromium bare-metal stent and a drug-eluting stent to its portfolio. According to the deal, Cordis plans to start selling Medinol’s NIRxcell BMS immediately and will exclusively distribute the company’s EluNir drug-eluting stent if it wins FDA […]
Medinol wins CE Mark for EluNir drug-eluting coronary stent
Medinol said today that it won CE Mark clearance for its EluNir drug-eluting stent – a device designed with a low metal footprint and an elastomeric coating. In one 958-patient clinical study, the coronary stent had a 12-month target lesion failure rate of 5.4% and a 0% rate of late stent thrombosis, proving non-inferior to […]