Cook Medical announced today that the U.S. Dept. of Defense (DOD) awarded it an ECAT contract for implantable medical devices. The contract includes implantable vascular medical devices like the Zilver PTX drug-eluting peripheral stent. It also applies to the Zenith aortic endografts and associated interventional devices for treating vascular disease. Cook Medical said in a […]
Stents
Abbott has positive data for Esprit drug-eluting resorbable scaffold
Abbott [WtwhTicker symbol=”ABT”](NYSE: ABT)[/WtwhTicker] today announced data supporting its Esprit BTK everolimus-eluting resorbable scaffold system. The company designed Esprit BTK to treat people with chronic limb-threatening ischemia (CLTI), a severe stage of peripheral artery disease (PAD). Abbott said its LIFE-BTK trial met both of its primary safety and effectiveness endpoints. It demonstrated the reduction of […]
FDA approves Medinol drug-eluting coronary stent
Medinol this week announced it received FDA approval for its Elunir-Perl drug-eluting stent (DES) for the treatment of coronary artery disease. Elunir-Perl is the latest evolution in the company’s Elunir DES product portfolio. It has four radiopaque markers, two at each end of the stent, as well as a hybrid polymer-metal radiopaque catheter tip. The […]
Boston Scientific hit with $42M verdict in drug-eluting stents IP case
Boston Scientific (NYSE:BSX) must pay $42 million in unpaid royalties to TissueGen and the University of Texas, a federal jury in Delaware has ruled. The jury verdict yesterday came after the finding that Boston Scientific’s Synergy BP drug-eluting stents infringed on TissueGen and the university’s IP. The patent under question arose out of research that […]
FDA approves Medtronic drug-eluting stents for treating bifurcation lesions
Medtronic (NYSE:MDT) announced today that the FDA approved its Onyx drug-eluting stents (DES) for treating certain lesions. Approval covers the treatment of non-left main bifurcation lesions utilizing the provisional bifurcation stenting technique. The technique uses a single stent to treat the bifurcation in percutaneous coronary interventions (PCIs). It applies to the FDA-approved and CE-marked Onyx […]
Medtronic launches Onyx Frontier drug-eluting stent
Medtronic (NYSE:MDT) launched its newest drug-eluting coronary stent, Onyx Frontier. The Fridley, Minnesota-based company designed the Onyx Frontier drug-eluting stent (DES) to build upon its Resolute Onyx DES. It uses the same stent platform as the Resolute Onyx and has an enhanced delivery system to increase acute performance in interventional coronary artery disease procedures. Get […]
Medtronic’s Resolute Onyx drug-eluting stent demonstrates strong safety, efficacy
Data presented today at the EuroPCR 2022 event supported the use of the Medtronic (NYSE:MDT) Resolute Onyx drug-eluting stent. University of Padua Medical School Head of Interventional Cardiology Dr. Giuseppe Tarantini presented late-breaking clinical trial results from the investigator-initiated, Medtronic-funded ROLEX (Revascularization Of LEft Main With Resolute onyX) registry. ROLEX, the largest real-world, multicenter prospective […]
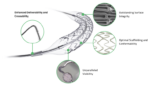
Ariste Medical co-founder sees great potential for drug-coated implants and orthopedics
It’s been more than a decade since Lisa Jennings launched not one but two companies in the Great Recession. In 2020, she sold CirQuest Labs to MLM Medical Labs, where Jennings serves as chief scientific officer and managing director of U.S. operations. More recently, her pre-commercial medtech development startup, Ariste Medical, won FDA 510(k) approval for […]
Ivantis to pay $60M in patent litigation settlement with Glaukos
Glaukos (NYSE:GKOS) announced today that it entered into a settlement with Ivantis to terminate a three-year-old patent infringement lawsuit. San Clemente, California-based Glaukos’ patent infringement lawsuit, initiated on April 14, 2018, in the U.S. District Court for the Central District of California, Southern Division, concerned Ivantis’ Hydrus Microstent for reducing eye pressure in glaucoma patients. […]
Glaukos submits supplemental PMA application for iStent Infinite
Glaukos (NYSE:GKOS) announced today that it submitted a supplemental premarket approval application to the FDA for its iStent Infinite system. San Clemente, California–based Glaukos designed the iStent Infinite trabecular micro-bypass system for use in a standalone procedure to reduce elevated intraocular pressure (IOP) in patients with open-angle glaucoma uncontrolled by prior surgery or medical therapy. […]