The FDA this week labeled Fresenius Kabi’s recall of some Ivenix Infusion Systems (IIS) as Class I, the most serious kind. Fresenius Kabi USA issued the recall of Ivenix Infusion System LVP software, its infusion pump software, due to an issue of multiple software anomalies occurring that can potentially result in serious patient harm or […]
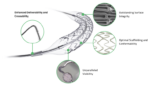
FDA approves Medinol drug-eluting coronary stent
Medinol this week announced it received FDA approval for its Elunir-Perl drug-eluting stent (DES) for the treatment of coronary artery disease. Elunir-Perl is the latest evolution in the company’s Elunir DES product portfolio. It has four radiopaque markers, two at each end of the stent, as well as a hybrid polymer-metal radiopaque catheter tip. The […]
B. Braun launches DoseTrac Enterprise infusion management software
B. Braun today announced it launched its next generation of infusion management software, called DoseTrac Enterprise. The new software allows organizations to receive a mix of real-time views and retrospective reporting features better to understand their infusion pump fleet and associated data. DoseTrac Enterprise can connect up to 40,000 pumps at an unlimited number of […]
Fresenius Kabi recall of Ivenix infusion systems in Class I
The FDA this week said the Fresenius Kabi recall of some Ivenix infusion systems is Class I, the most serious kind. Fresenius Kabi is recalling some Ivenix infusion systems due to a leak in the system that allows fluid to enter the administration set loading area near the air detector. The leak could damage the electrical system […]
Fresenius Kabi wins Vizient contract for Ivenix infusion system
Fresenius Kabi this week announced its Ivenix infusion system was awarded an Innovative Technology contract from Vizient. Vizient members include more than half of all acute care hospitals in the U.S., and the Innovative Technology contracts are recommended after review and interaction with products submitted through the organization’s program. The contract sets terms for hospitals […]
Surmodics announces regulatory delay for its SurVeil balloon
The FDA noted more information on biocompatibility and labeling is needed.
The FDA has indicated that Surmodics’ Surveil drug-coated balloon premarket approval application is not approvable. According to an FDA letter to the company, Surmodics’ Surveil was not approved due to certain information in the biocompatibility and labeling categories. The governing agency said more information needs to be added by an amendment to Surmodics’ PMA application to […]
Ascensia Diabetes integrates its Eversense CGM with Apple Health
Ascensia Diabetes Care announced today that it has updated its Eversense Continuous Glucose Monitoring system app to integrate with Apple Health. The Parsippany, N.J.-based company makes the Contour Blood Glucose Monitoring (BGM) system and distributes Senseonics’ Eversense CGM system. The new Apple Health integration will allow people with diabetes using Senseonics’ Eversense CGM systems to […]
Medtronic launches Onyx Frontier drug-eluting stent
Medtronic (NYSE:MDT) launched its newest drug-eluting coronary stent, Onyx Frontier. The Fridley, Minnesota-based company designed the Onyx Frontier drug-eluting stent (DES) to build upon its Resolute Onyx DES. It uses the same stent platform as the Resolute Onyx and has an enhanced delivery system to increase acute performance in interventional coronary artery disease procedures. Get […]
Retractable Technologies downsizes workforce
Retractable Technologies this week announced that it has reduced its workforce by 16% due to the completion of its facility expansion efforts — and the completion of U.S. government orders related to COVID-19 vaccines. The staff reduction will affect the Little Elm, Texas-based company’s production, operations and logistics departments. Retractable Technologies said in an SEC filing that there […]
FDA approves GE Healthcare software that automates anesthesia, reduces greenhouse gas emissions during surgery
GE Healthcare recently announced that it received FDA premarket approval for its End-tidal (Et) Control software for general anesthesia delivery. The software is cleared for use with GE Healthcare’s Aisys CS2 anesthesia delivery system. The Chicago-based company initially released the technology in Europe in 2010 and is currently used in over 100 countries. Get the full […]