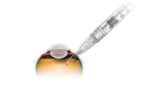
 Roche‘s (OTC:RHHBY) Genentech touted additional positive data last week from a Phase II study of its Port Delivery System, a refillable eye implant designed to deliver ranibizumab to treat wet age-related macular degeneration.
Roche‘s (OTC:RHHBY) Genentech touted additional positive data last week from a Phase II study of its Port Delivery System, a refillable eye implant designed to deliver ranibizumab to treat wet age-related macular degeneration.
The company first reported top-line results from the Phase II Ladder study in July. The implant is intended to allow wet-AMD patients to go for months without needing to see an ophthalmologist for treatment; the current standard of care for wet-AMD patients involves monthly injections of therapy.
Genentech touted that the majority of people enrolled in its Phase II Ladder trial went at least six months between receiving the device and the first refill.
The company also found that people in the high-dose PDS group experienced similar vision outcomes as those who received o.5 mg of ranibizumab every four weeks.
Also last week, Genentech revealed the design of its Phase III Archway trial, which is intended to assess the PDS implant with ranibizumab compared to monthly ranibizumab injections in 360 people with wet age-related macular degeneration.
The trial’s primary endpoint is the change from baseline in best-corrected visual acuity score at the average of week 36 and week 40, according to Genentech. The study, which is actively enrolling participants, is slated to wrap up by May of 2022.

