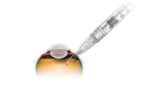
Genentech announced today that it received FDA approval for its Susvimo injection for treating macular degeneration (AMD). South San Francisco-based Genentech, a member of the Roche Group, designed its Susvimo ranibizumab injection (100 mg/mL) for intravitreal use via ocular implant for treating people with wet, or neovascular, age-related macular degeneration (AMD) who have previously responded […]
Genentech
FDA accepts biologics license application for Genentech’s drug-eluting eye implant
Genentech announced that the FDA accepted its Biologics License Application (BLA) for its Port Delivery System (PDS) with ranibizumab. The PDS is a permanent, refillable eye implant that’s the approximate size of a grain of rice. It offers continuous delivery of a customized formulation of ranibizumab over a period of months to reduce the treatment […]
Genentech touts study results for spinal muscular atrophy drug in babies
Genentech today touted two-year data evaluating the use of its Evrysdi therapeutic in infants with symptomatic Type 1 spinal muscular atrophy (SMA). South San Francisco, Calif.-based Genentech’s Firefish Phase 2/3 global study for Evrysdi (risdiplam) in infants between 1-7 months old showed that the orally administered (liquid or feeding tube) therapeutic continued to improve motor […]
Genentech wins FDA approval for Xolair prefilled syringe
Genentech announced today that the FDA approved its supplemental biologics license application for the Xolair prefilled syringe. South San Francisco-based Genentech touts the Xolair (omalizumab) prefilled syringe for self-injection across all approved U.S. indications as the only FDA-approved biologic designed to target and block immunoglobulin E (IgE) for treating moderate to severe persistent allergic asthma, […]
Enable Injections inks deal with Genentech
Enable Injections said yesterday that it entered a developmental partnership with Genentech for the Enable enFuse device. Cincinnati-based Enable’s enFuse is an on-body drug delivery platform containing a drug transfer system compatible with syringes and vial containers. Currently, the enFuse is being developed for subcutaneous delivery of large volumes potentially rising to 50 mL. The […]
FDA approves Genentech’s prefilled autoinjector for Actemra
Roche‘s (OTC:RHHBY) Genentech said today that the FDA approved its ACTPen single-dose prefilled autoinjector for Actemra as a new formulation for adults with moderate to severe active rheumatoid arthritis and for adults with giant cell arteritis. The U.S. regulatory agency also approved the device for administration by caregivers to patients as young as two years old […]
Genentech touts data for refillable eye implant in wet-AMD trial
Roche‘s (OTC:RHHBY) Genentech touted additional positive data last week from a Phase II study of its Port Delivery System, a refillable eye implant designed to deliver ranibizumab to treat wet age-related macular degeneration. The company first reported top-line results from the Phase II Ladder study in July. The implant is intended to allow wet-AMD patients to go […]
Genentech kicks off Phase III trial for eye implant
Roche‘s (OTC:RHHBY) Genentech reported this week that the company launched Phase III trials evaluating its Port drug-delivery implant in patients with wet age-related macular degeneration and the drug faricimab in patients with diabetic macular edema. The company’s Port device is a refillable eye implant designed to continuously release drugs over a period of several months. “Wet […]
Genentech wins FDA nod for subcutaneous formulation of arthritis drug
Roche‘s (OTC:RHHBY) Genentech reported this week that the FDA approved the subcutaneous formulation of tocilizumab for the treatment of active systemic juvenile idiopathic arthritis in patients two years and older. The U.S. agency approved the intravenous formulation of Actemra for the same indication in 2011. Systemic juvenile idiopathic arthritis affects roughly 30,000 children in the U.S., according […]
Genentech touts positive data for tiny, refillable eye implant
Roche‘s (OTC:RHHBY) Genentech touted positive top-line data today from a Phase II study of its Port Delivery System, an eye implant designed to administer a sustained dose of ranibizumab in patients with wet age-related macular degeneration. The refillable device, which is slightly longer than a grain of rice, is intended to enable wet-AMD patients to go for […]