(Image from Lyra Therapeutics)
Lyra Therapeutics (NSDQ:LYRA) announced today that it initiated the Phase 3 Enlighten I trial for its LYR-210 treatment.
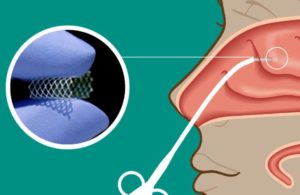
Watertown, Massachusetts-based Lyra’s Phase 3 Enlighten I clinical trial will evaluate LYR-210, delivered through Lyra’s proprietary XTreo drug-eluting implant, in adult, surgically naïve chronic rhinosinusitis (CRS) patients. The company designed LYR-210 for delivery in a brief, non-invasive, in-office procedure with up to six months of continuous anti-inflammatory medication in the sinonasal passages.
“LYR-210 is the first product candidate designed to provide six months of continuous therapy with a single treatment and represents a significant opportunity to introduce a new standard of care to the millions of CRS patients suffering with the disease and seeking a non-surgical treatment that provides significant symptom relief,” Lyra President & CEO Maria Palasis said in a news release. “Following the positive results achieved in the Lantern Phase 2 study of LYR-210 demonstrating rapid, durable, and clinically meaningful improvement, we look forward to advancing our Phase 3 Enlighten pivotal program with the initiation of Enlighten I, followed by initiation of Enlighten II, the second Phase 3 study, within the first half of this year.”
The Phase 3, multicenter, randomized, controlled trial will evaluate the efficacy and safety of LYR-210 compared to control with approximately 180 CRS patients to be enrolled and randomized 2:1 to receive LYR-210 or the control.
Lyra said the primary endpoint is the change from baseline in a composite score of three cardinal symptoms (3CS) of CRS (i.e., nasal blockage, nasal discharge, and facial pain) at week 24. After 24 weeks, control patients in Enlighten I will be eligible to cross over into treatment as part of an extension study that will include repeat administration in previously treated patients. The company expects to complete enrollment in the first half of 2023.
The company’s second product candidate, LYR-220, also had a trial initiated. The Beacon Phase 2 trial will evaluate safety, tolerability, pharmacokinetics and efficacy in two designs of LYR-220 in adult patients with CRS who had prior surgery for their CRS symptoms. The trial of approximately 70 symptomatic adult CRS subjects who had a prior bilateral sinus surgery will take place over 24 weeks, with enrollment expected to be completed at the end of 2022.
“As a practicing rhinologist, I am very optimistic that these product candidates will provide meaningful improvement for the millions of CRS sufferers who currently have no approved therapy and am encouraged to see the forward progress of these product candidates through clinical development,” Lyra CMO Dr. Robert Kern said. “I believe these innovative treatments have the potential to transform the current treatment paradigm for the broad spectrum of CRS patients who have failed first-line medical management.”

