(Image from Lyra Therapeutics)
Lyra Therapeutics (NSDQ:LYRA) is touting preclinical data for its XTreo drug-eluting implant for dosing in local sinus tissues.
Watertown, Massachusetts-based Lyra’s preclinical results were published — “Drug Release and Pharmacokinetic Evaluation of Novel Implantable Mometasone Furoate Matrices in Rabbit Maxillary Sinuses” — were published online in the peer-review journal, American Journal of Rhinology & Allergy.
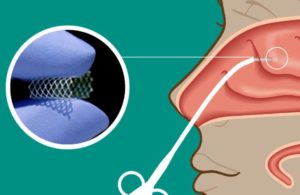
The pharmacokinetics and drug release study evaluated the release of mometasone furoate (MF), a potent anti-inflammatory corticosteroid formulated into Lyra’s proprietary XTreo matrix in a rabbit model, according to a news release. XTreo, comprised of a flexible mesh capable of taking a high payload of a drug, provides a consistent release of the therapeutic over time, with Lyra touting capabilities of six months of drug delivery.
Lyra said the results demonstrate that XTreo MF provides targeted, sustained and efficient dosing to local sinus tissues that is superior to intranasal corticosteroid sprays (INCS).
In the study, XTreo matrices self-expanded upon deployment to conform to the irregular geometry of the maxillary sinus cavities and provided a sustained release of MF in vitro and in vivo for two MF matrices of distinct release durations. Low levels of MF were detected in plasma, demonstrating XTreo’s ability to maximize the therapeutic effect to the immediately contacted tissues and potentially eliminate systemic side effects.
“The outcomes from this study supported Lyra’s advancement into clinical development for the first application of our novel XTreo platform, LYR-210 for the treatment of Chronic Rhinosinusitis (CRS), which is now poised to enter Phase 3 studies,” Lyra president & CEO Maria Palasis said in the release. “XTreo is a powerful drug delivery platform that can target a precise dose of a therapeutic agent consistently over an extended period of time, up to many months. These characteristics translate into multiple potential benefits, including increased efficacy, elimination of patient adherence issues, and avoidance of systemic side effects. We believe our proprietary platform technology can provide optimal treatment for CRS, as well as other chronic ear, nose and throat (ENT) diseases.”

