Tightly-packed endothelial cells line blood vessels and maintain a highly structured barrier. But researchers from Rice University, Emory University and the Georgia Institute of Technology have found a way to selectively create “leaky” blood vessels for targeted drug delivery.
The team’s study, which was published in Nature Communications, reported that magnets can help lead iron-oxide nanoparticles into endothelial cells, as well as create gaps and then close them.
This method could allow large-molecule drugs to reach deep tissues that traditional therapies can’t get to, according to Rice bioengineer Gang Bao.
“For many diseases, systemic delivery through the blood stream is the only way to deliver molecules to the site,” Bao said in prepared remarks. “Small molecules can penetrate the blood vessel and get into the diseased cells, but large molecules like proteins or drug-loaded nanoparticles cannot pass the endothelium effectively unless it is leaky.”
Blood vessels that feed cancerous tumors often have holes in the endothelial lining, but they don’t seal on demand.
Bao also hopes to use magnets to deliver nanoparticle-stem cell combinations to damaged tissue.
“Unless you can do direct injection of stem cells, let’s say into the heart, you have to do systemic delivery and you have no control over where they go,” Bao said.
“Our initial idea was to deliver magnetic nanoparticles into stem cells and then use a magnet to attract the stem cells to a particular location. In doing so, we also discovered that by applying a magnetic field, we could generate changes in the cell’s skeletal structure in terms of the actin filament structures.”
Structural characteristics of cells give them their shape, keeping the cells compact within the endothelial barrier. The team hypothesized that a magnetic field would distort structural elements of cells and create gaps in the cells’ distribution.
“We thought if we could alter the cell-cell junction by using magnetic force, there was a possibility that we could engineer the leakiness of the vessel,” Bao said.
The researchers developed a microfluidic flow chamber to model the vascular system and lined the tubes with endothelial cells.
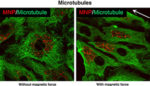
Imaging revealed that fluorescent-tagged nanoparticles were distributed evenly among the endothelial barrier without a magnetic field. When the team applied a magnetic field, the particles redistributed and created gaps among the cells.
Most of the gaps closed after 12 hours once the force was relaxed, according to the team.
“It’s a pretty dramatic change,” Bao said. “Once you apply the force, given enough time, the structure of the cells changes. That leads to the opening of the cell-cell junction.”
Bao cautioned that to take this method to a clinical setting, researchers would need a large device to generate a magnetic field required to treat the heart or liver.
“We don’t have that yet,” he said. “To drive this to a clinical setting will be a challenge.”

