 Traditional implants in the gastrointestinal tract are meant to pass through a patient’s system, delivering a drug in short bursts or recording the health of a patient’s colon.
Traditional implants in the gastrointestinal tract are meant to pass through a patient’s system, delivering a drug in short bursts or recording the health of a patient’s colon.
But in recent years, scientists have sought after ultra-long lasting ingestible devices that can deliver drugs for several weeks in a patient’s GI tract. Lyndra, Inc., a start-up based on such technology from the Massachusetts Institute of Technology, raised $23 million in April to support development of its star-shaped capsule.
Sustained-release drug delivery devices that reside in the gastrointestinal tract require a power source and, as gastroenterologist Giovanni Traverso told Drug Delivery Business News, batteries don’t make the cut.
“When you’re talking about keeping something in the body for a long period of time, and you want to power for a long period of time, batteries really are insufficient, because they have a finite amount of energy,” he said.
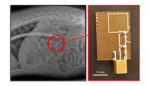
Traverso, based at Brigham & Women’s Hospital, and a group from Draper teamed up to demonstrate a method that could wirelessly power an ingestible drug delivery device.
The team decided to explore midfield transmission as a way to transfer power between an antenna outside of the body to another antenna inside a patient’s digestive tract. They tested their technique in a simulation, in live tissue and in a pig model. Using this method, the team delivered 100 to 200 microwatts of power to their device, which is suitable for small electronics.
In a pig model, the external antenna was able to transfer power from 2 to 10 centimeters away. The team reported that the energy transfer did not cause any tissue damage.
Draper researcher Brian Smith said the GI tract has a host of interesting characteristics that they catered to while developing an implantable electronic system – any device that resides in the GI tract has to be able to stand up to the stomach’s harsh, acidic environment.
“So that precludes the use of a lot of materials that would corrode, or degrade, in that environment, but it also opens the field to larger devices,” he said. “The GI tract is interesting, in that we have more space and it doesn’t have the same sort of aspects of implantation that other electronic systems in the body have.”
Smith added that the team had to consider the relationship between the size of the antennas and the size of the device.
“And that’s a big challenge for any sort of implantable or ingestible system. So it’s the combination of the demands of the electronic system and the ability of the biological system to accept it.”
Looking ahead, the team plans to devise a way to trigger the device to fall apart and pass through a patient’s system. But Traverso pointed out that a wirelessly-powered ingestible device could have a number of medical applications, from sensing to drug delivery.
“What we see is someday having systems that you could swallow and they could sense and deliver treatments, either on demand or secondary to a specific physiological cue.”

