Lyndra Therapeutics yesterday announced positive results from a Phase 2 study of its once-weekly, oral schizophrenia treatment. Watertown, Mass.-based Lyndra’s oral, long-acting, extended-release risperidone capsule, LYN-005, is designed for the weekly treatment of schizophrenia. Data from the company’s first repeat-dose Phase 2 study demonstrate that LYN-005 offered sustained therapeutic levels of risperidone over the one-week […]
lyndra
Lyndra Therapeutics appoints Jenkins, Fernandes to executive roles
Lyndra Therapeutics announced today that it appointed Abigail Jenkins and Jacqueline Fernandes to executive positions at the company. Jenkins will take over as chief commercial & business officer, while Fernandes assumes the role of VP of data compliance and information technology at the Watertown, Mass.-based developer of oral, long-acting, sustained-release therapeutics. “We are pleased to […]
Lyndra to develop ultra-long-acting opioid use disorder therapy
Lyndra Therapeutics (Watertown, Mass.) announced that it is developing an ultra-long-acting oral treatment for opioid use disorder. On average, about 130 Americans die every day from an opioid overdose, according to the Centers for Disease Control and Prevention. Lyndra Therapeutics received a grant from the National Institutes of Health to help find new treatment strategies […]
Lyndra raises $55m for long-acting drug delivery capsule
Lyndra Therapeutics said today that it raised $55 million in a Series B round to support the development of its oral drug delivery system. All of the investors from the company’s $23 million Series A round resubscribed for the Series B round, Lyndra reported. New investors included the Bill & Melinda Gates Foundation. Lyndra’s oral […]
Lyndra touts positive results for long-acting drug-delivery tech
Lyndra Therapeutics reported positive results from a pharmacokinetics study of its once-weekly drug-delivery capsule, touting that it successfully transitioned a once-daily therapy to a weekly dosage form. The Watertown, Mass.-based company’s oral drug-delivery technique is designed to provide sustained release of one or more drugs for up to a week. Lyndra’s star-shaped capsule opens when […]
Researchers develop capsule to deliver a week’s worth of HIV drugs
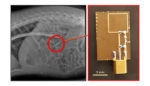
A team of researchers from the Massachusetts Institute of Technology and Brigham & Women’s Hospital have developed a drug-delivery capsule that can deliver a week’s worth of HIV drugs in a single dose. The technology is designed to make it easier for people with HIV to deal with the strict schedule of medications they need […]
Ocular continues personnel shakeup with new CMO | Personnel Moves – Sept. 13, 2017
After Ocular Therapeutix‘s (NSDQ:OCUL) founder & CEO, Amar Swahney, stepped down earlier this year, pharma executive Antony Mattessich stepped in to assume the corner office. He signaled some major changes to the company’s business strategy in a call with analysts last month, including an attempt to ‘aggressively seek new partnerships’ and ‘enhance [the company’s] credibility’. As […]
Lyndra lands $105m deal with Allergan for once-weekly Alzheimer’s drugs
Boston-based startup Lyndra said today that it is partnering with pharma giant Allergan (NYSE:AGN) to develop orally administered, once-weekly products for the treatment of Alzheimer’s disease. The collaboration will combine Allergan’s proprietary Alzheimer’s drugs with Lyndra’s sustained-release technology, which is designed to temporarily reside in the stomach for up to one week while delivering a drug. […]
Powering the next generation of ingestible drug delivery devices
Traditional implants in the gastrointestinal tract are meant to pass through a patient’s system, delivering a drug in short bursts or recording the health of a patient’s colon. But in recent years, scientists have sought after ultra-long lasting ingestible devices that can deliver drugs for several weeks in a patient’s GI tract. Lyndra, Inc., a start-up […]
Lyndra gets boost for drug delivery platform from U.S. allergy institute
Lyndra said today that it won a 5-year grant from the National Institute of Allergy and Infectious Diseases, a branch of the National Institutes of Health. The grant is slated to support the formulation and preclinical development of a once-weekly oral HIV treatment. The Watertown, Mass.-based company makes use of a sustained-release technology developed by Robert […]