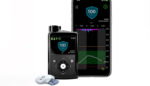
Medtronic (NYSE:MDT) announced positive real-world data supporting its next-generation MiniMed 780G insulin pump system. Results from real-world analyses in Europe and Chile, along with clinical data, were presented by Medtronic at the American Diabetes Association (ADA) 82nd Scientific Sessions in New Orleans. According to a news release, the MiniMed 780G advanced hybrid closed loop system, […]
Medtronic
FDA approves Medtronic’s In.Pact 018 drug-coated balloon catheter
Medtronic (NYSE:MDT) announced that it received FDA approval for its In.Pact 018 paclitaxel-coated balloon catheter. Fridley, Minnesota-based Medtronic’s In.Pact 018 paclitaxel-coated percutaneous transluminal angioplasty (PTA) balloon catheter, a drug-coated balloon product, received an indication for the interventional treatment of peripheral arterial disease (PAD) in the superficial femoral and popliteal arteries. According to a news release, […]
Medtronic ‘hopeful’ on FDA clearance for next-gen diabetes tech in FY 2023
The Medtronic (NYSE:MDT) diabetes business sputtered in the fourth quarter but could be bolstered by a major regulatory nod this year. The Fridley, Minnesota-based company saw revenues for its diabetes arm fall by 7.7% year-over-year in the fourth quarter amid overall misses on both sales and adjusted earnings per share compared to Wall Street’s expectations. […]
Medtronic’s Resolute Onyx drug-eluting stent demonstrates strong safety, efficacy
Data presented today at the EuroPCR 2022 event supported the use of the Medtronic (NYSE:MDT) Resolute Onyx drug-eluting stent. University of Padua Medical School Head of Interventional Cardiology Dr. Giuseppe Tarantini presented late-breaking clinical trial results from the investigator-initiated, Medtronic-funded ROLEX (Revascularization Of LEft Main With Resolute onyX) registry. ROLEX, the largest real-world, multicenter prospective […]
FDA approves Medtronic’s Onyx Frontier drug-eluting coronary stent
Medtronic (NYSE:MDT) announced today that it received FDA approval for its Onyx Frontier drug-eluting stent (DES). Onyx Frontier, the latest iteration of Medtronic’s Resolute DES family, was designed to leverage the same stent platform as Resolute with an enhanced delivery system for improving deliverability and increasing acute performance, even in the most challenging of cases. […]
Medtronic completes Intersect ENT acquisition
Medtronic (NYSE:MDT) announced that it completed its previously announced $1.1 billion acquisition of Intersect ENT (Nasdaq:XENT). Fridley, Minnesota-based Medtronic announced in August 2021 that it would acquire all outstanding shares of the Menlo Park, Calif.-based sinus implant maker for $28.25 per share in an all-cash transaction. As a result of the transaction, Medtronic acquires Intersect ENT’s Propel and Sinuva drug-eluting […]
CGMs, insulin pumps and more: the biggest diabetes news to come out of ATTD 2022
All kinds of technologies were on display at the Advanced Technologies & Treatments for Diabetes (ATTD) conference in Barcelona last week. From continuous glucose monitoring (CGM) to automated insulin delivery, with plenty in between, a number of companies shared positive data for their diabetes offerings. Here are five of the biggest stories to come out […]
Study shows sustained improvements with Medtronic’s MiniMed 780G with Guardian 4
Medtronic (NYSE:MDT) announced today that the extended study phase of its MiniMed 780G insulin pump system produced positive data. Fridley, Minnesota-based Medtronic presented new data from the study of MiniMed 780G with the no-calibration, non-adjunctive Guardian 4 sensor at the Advanced Technologies & Treatments for Diabetes (ATTD) Conference in Barcelona. Data demonstrated sustained improvements in […]
Analysts see Medtronic’s diabetes, spine businesses as most likely spinoff candidates
Needham analysts published a report singling out the diabetes and spine businesses as possible spinoff options for Medtronic (NYSE:MDT). Mike Matson, David Saxon and Joseph Conway of Needham pointed to previous comments from Medtronic management that indicate the potential for changes in the coming year, with a spinoff of a large business within Medtronic considered to be […]
Medtronic issues voluntary recall for certain In.Pact drug-coated balloon catheters
Medtronic (NYSE:MDT) announced today that it voluntarily recalled a subset of its In.Pact Admiral and In.Pact AV balloon catheters. The company initiated the recent recall of the paclitaxel-coated percutaneous transluminal angioplasty (PTA) balloon catheters due to the potential for pouch damage resulting in a loss of sterility. According to the company, approximately 6,000 In.Pact Admiral […]