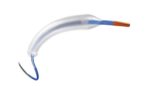
Medtronic (NYSE:MDT) announced today that it received FDA approval for its Onyx Frontier drug-eluting stent (DES). Onyx Frontier, the latest iteration of Medtronic’s Resolute DES family, was designed to leverage the same stent platform as Resolute with an enhanced delivery system for improving deliverability and increasing acute performance, even in the most challenging of cases. […]
Cardiovascular
Abbott announces availability of Xience Skypoint drug-eluting stent in extended sizes
Abbott (NYSE:ABT) announced today that its Xience Skypoint drug-eluting stent platform is now available globally. Xience Skypoint has received FDA approval, the CE mark, and PMDA approval in Japan for the broadest expansion with the availability of extended sizes of 4.5 mm and 5 mm offerings. According to Abbott, Xience Skypoint stents are now the […]
Medtronic issues voluntary recall for certain In.Pact drug-coated balloon catheters
Medtronic (NYSE:MDT) announced today that it voluntarily recalled a subset of its In.Pact Admiral and In.Pact AV balloon catheters. The company initiated the recent recall of the paclitaxel-coated percutaneous transluminal angioplasty (PTA) balloon catheters due to the potential for pouch damage resulting in a loss of sterility. According to the company, approximately 6,000 In.Pact Admiral […]
MedAlliance touts 18-month data for treating lesions with sirolimus-eluting balloon
MedAlliance today touted late-breaking clinical trial data demonstrating positive outcomes with its Selution SLR drug-eluting balloon. The company presented 18-month results from its Prestige below-the-knee (BTK) study evaluating the safety and performance of Selution SLR in treating long tibial occlusive lesions (TASC C & D) in patients with critical limb ischemia (CLI) at VIVA21. According to a […]
MedAlliance completes trial enrollment for sirolimus-eluting balloon for treating PAD
MedAlliance announced today that it completed patient enrollment for a trial of its Selution SLR sirolimus drug-eluting balloon. Geneva, Switzerland-based MedAlliance, along with Japanese partner MDK Medical, completed the enrollment following the acceptance of a Clinical Trial Notification (CTN) by the Pharmaceutical Products and Medical Devices Agency (PMDA) of Japan in June 2020. The study […]
FDA approves Baxter’s norepinephrine injection for treating low blood pressure
Baxter (NYSE:BAX) announced today that it received FDA approval for its premix norepinephrine bitartrate in 5% dextrose injection. Deerfield, Illinois-based Baxter launched the norepinephrine injection following the approval for raising blood pressure in adult patients with severe, acute hypotension (low blood pressure). According to a news release, the company’s formulation of norepinephrine is the first […]
Sinomed launches HT Supreme drug-eluting stent in Europe
Sinomed has completed the first commercial implantation of its HT Supreme drug-eluting stent in Ireland. Faisal Sharif, professor of translational cardiovascular medicine and innovation at the National University of Ireland Galway, successfully performed the first procedure with HT Supreme, a healing-targeted drug-eluting stent designed to treat patients with narrowing or blockages to their coronary arteries. […]
Abbott CMO Dr. Nick West touts latest iteration of drug-eluting stent
Abbott executive Dr. Nick West believes the company offers the “best-in-class” drug-eluting stent with its Xience platform. When Abbott (NYSE:ABT) CMO & divisional VP of global medical affairs for its vascular business Dr. Nick West looks back at his use of drug-eluting stents in the early 2000s, all he sees now is innovation. As a practicing interventional cardiologist, West […]
Tenaya Therapeutics prices $180M IPO for targeted therapies
Tenaya Therapeutics announced that it priced an underwritten, upsized initial public offering of common stock worth $180 million. South San Francisco-based Tenaya develops therapies for rare generic disorders and more prevalent heart conditions through gene therapy, cellular regeneration and precision medicine. The company is actively exploring different routs of administration and different infusion- and injection-based methods […]
Boston Scientific initiates coronary drug-coated balloon study in U.S.
Boston Scientific (NYSE:BSX) this week launched its Agent IDE trial for its Agent drug-coated balloon. The U.S. prospective, randomized clinical trial will evaluate the safety and effectiveness of a drug-coated balloon (DCB) in patients with coronary in-stent restenosis in lesions up to 26 mm in length in a coronary artery 2.0 mm to 4.0 mm in […]