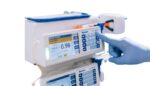
Baxter (NYSE:BAX) has issued a letter to affected customers recommending a correction for its Novum IQ infusion pump systems. The correction applies to all Novum IQ large volume pumps (LVP) and syringe pumps (SP). Baxter said users should consider alternate pumps if available for care involving high-risk medications and/or critical illness. However, customers can continue […]
Food & Drug Administration (FDA)
Glucotrack to submit long-term CGM implant for FDA IDE this year
Glucotrack (Nasdaq:GCTK) today announced multiple developments in the timeline for its long-term continuous blood glucose monitor (CBGM). The Rutherford, New Jersey-based company plans to file an FDA investigational device exemption (IDE) submission in the fourth quarter of this year. It also remains on track to implant the first patients in a long-term, multi-center feasibility study […]
Sinocare withdraws FDA submission for CGM to focus on next-gen system
Sinocare announced recently that it withdrew its FDA submission for the first-generation iCan i3 continuous glucose monitor (CGM). The company withdrew the FDA 510(k) submission as it looks to instead expedite bringing its next-gen solution to the market. China-based Sinocare designed the iCan i3 CGM for ease of use, with no scanning, no fingersticks and […]
FDA warns on Baxter infusion pump issue that potentially led to deaths
The FDA issued an early alert related to an issue with Novum IQ large-volume infusion pumps made by Baxter (NYSE:BAX). This notice comes just weeks after an initial warning about potential underinfusion with all Novum IQ large-volume pumps. That alert included reports of one serious injury and no deaths associated with the issue. Now, the FDA […]
Dexcom updates CGM receiver recall that led to serious adverse events
Dexcom (Nasdaq:DXCM) has issued an update related to a recall for its continuous glucose monitor (CGM) components. Last month, the FDA disclosed Dexcom’s recalls related to its Dexcom One and One+ offerings and the G6 and G7 CGMs. The One and One+ systems, which are mainly the company’s commercial CGM offering outside the U.S., feature the established G6 and G7 sensors […]
Johnson & Johnson wins FDA priority review for drug delivery system
Johnson & Johnson (NYSE:JNJ) announced today that the FDA granted priority review to a new drug application (NDA) for its TAR-200. TAR-200, an intravesical gemcitabine-releasing system, treats patients with Bacillus Calmette-Guérin (BCG)-unresponsive high-risk non-muscle invasive bladder cancer (HR-NMIBC) with carcinoma in situ (CIS), with or without papillary tumors. A healthcare professional places TAR-200 into the […]
BD issues voluntary recall on certain Alaris infusion pump modules
BD (NYSE:BDX) announced a voluntary recall related to certain Alaris and Alaris infusion pump modules. Affected Alaris products may have been serviced with previously recalled bezel kit assemblies. BD in 2019 recalled those kit assemblies manufactured between April 2011 and June 2017. They should not be used for service with the Alaris and Alaris Pump […]
Convatec wins FDA approval for Parkinson’s treatment infusion tech
Convatec announced today that it received FDA approval for the delivery of a Parkinson’s disease therapy through its Neria Guard infusion set. The FDA approved apomorphine hydrochloride for subcutaneous infusion for the treatment of Parkinson’s. London-based Convatec’s Neria Guard set delivers the therapy under the skin (subcutaneous) as a continuous infusion via a small portable […]
Next-gen Medtronic insulin pump reportedly set for FDA submission, patch pump on the way
BTIG analysts said today that MiniMed, the Medtronic (NYSE:MDT) diabetes business, has some significant pipeline updates. Marie Thibault, Sam Eiber and Alexandra Pang shared notes from their meeting with Que Dallara, soon-to-be CEO of the to-be separated Diabetes unit, at the American Diabetes Association’s 85th Scientific Sessions in Chicago. Among those updates, the company expects […]
Lilly to submit once-weekly insulin for regulatory review this year following strong clinical results
Eli Lilly (NYSE:LLY) today announced positive safety and efficacy data for its investigational once-weekly insulin efsitora alfa (efsitora). The QWINT-1, QWINT-3 and QWINT-4 Phase 3 clinical trials evaluated efsitora in adults with type 2 diabetes who used insulin for the first time, previously used daily basal insulin, and previously used daily basal insulin and mealtime insulin, […]