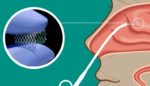
Lyra Therapeutics (Nasdaq:LYRA) announced today that it appointed Dr. Richard Nieman as its new chief medical officer (CMO). Nieman’s appointment as CMO of Lyra, which develops the XTreo — a platform for enabling precise, sustained and local delivery of medications to the ear, nose and throat (ENT) passages and other diseased tissues — will be […]
Respiratory
Lyra Therapeutics closes $100.5M private placement
Lyra Therapeutics (Nasdaq:LYRA) announced today that it closed its previously announced private placement worth $100.5 million. The company announced last week that, in the private placement, investors had the option in the private placement to purchase either shares of Lyra’s common stock at $4.22 per share or, in lieu thereof, pre-funded warrants to purchase shares […]
Lyra Therapeutics announces $100.5M private placement
Lyra Therapeutics (Nasdaq:LYRA) announced today that it agreed to sell securities in a private placement worth more than $100 million. The company — which develops the XTreo platform designed to enable precise, sustained, and local delivery of medications to the ear, nose and throat (ENT) passages and other diseased tissues — did not list an […]
Viatris, Kindeva win FDA approval for first generic version of Symbicort inhalation aerosol
Viatris (Nasdaq:VTRS) and Kindeva Drug Delivery announced today that the FDA approved its generic version of AstraZeneca’s Symbicort. In March 2021, the FDA granted tentative approval to Breyna (Budesonide and Formoterol Fumarate Dihydrate Inhalation Aerosol) — the first generic version of Symbicort. Today, the agency granted approval for the abbreviated new drug application (ANDA) for […]
Teva rises on mixed-bag Q4, sets 2022 guidance
Teva Pharmaceuticals (NYSE:TEVA) shares ticked up this morning on fourth-quarter results that topped the consensus EPS forecast. The Tel Aviv, Israel–based company posted losses of $159 million, or 14¢ per share, on sales of $4.1 billion for the three months ended Dec. 31, 2021, for a bottom-line slide into the red on a sales decline […]
FDA grants new drug approval to Glenmark’s rhinitis-treating nasal spray
Glenmark Pharmaceuticals announced today that it received FDA approval for a new drug application (NDA) for Ryaltrais. Ryaltris, a fixed-dosed, prescription drug product nasal spray treats symptoms of seasonal allergic rhinitis in adults and pediatric patients over 12 years of age in the U.S. Glenmark’s fully owned subsidiary, Glenmark Specialty S.A. (Switzerland), received the NDA […]
Your favorite articles from Drug Delivery Business News in 2021
From continuous glucose monitors to insulin pens, plus needle-free injection devices and inhalers — here are the most popular articles on Drug Delivery Business News from 2021: Links to your favorite articles: Dexcom CEO Kevin Sayer says G7 will be ‘wonderful’ Eli Lilly partners with four companies on insulin pen tech Scancell picks PharmaJet’s needle-free […]
Inhaled drug delivery tech company Aerami to go public through SPAC merger
Aerami Therapeutics announced today that it entered into a merger agreement with FoxWayne Enterprises Acquisition Corp. (NSDQ:FOXWU). Through the definitive business combination agreement with the special purpose acquisition company (SPAC), Aerami, a developer of inhaled therapies for treating respiratory and chronic diseases, will be listed on the Nasdaq market as “Aerami Therapeutics Holdings, Inc.” According […]
Teva announces two offerings worth $7.5 billion, dips on missed Q3 projections
Teva Pharmaceuticals (NYSE:TEVA) shares took a hit today on third-quarter results that fell short of the consensus forecast. TEVA shares were down -4.7% at $8.95 per share in mid-morning trading. MassDevice’s MedTech 100 Index — which includes stocks of the world’s largest medical device companies — was down -0.8%. The Tel Aviv, Israel-based company posted […]
Philip Morris’ $1.2B offer for inhaled drug delivery tech developer becomes unconditional
Philip Morris (NYSE:PM) announced today that its offer to buy inhaled drug delivery technology developer Vectura has become unconditional. Having received valid acceptances for or acquired 74.77% of Vectura shares, in excess of the 50% required under the acceptance condition, along with confirming that all other conditions to the offer have been satisfied or waived, […]