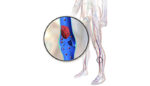
Medtronic (NYSE:MDT) said today that it launched a clinical trial to evaluate the use of its In.Pact paclitaxel-coated percutaneous transluminal angioplasty balloon catheter in patients with below-the-knee peripheral arterial disease. The company treated its 1st patient in the In.Pact BTK study, performing the procedure on a patient with critical limb ischemia. “The 1st patient enrollment in […]
Vascular
Study evaluates drug-device combo therapy for deep vein thrombosis
A National Institutes of Health-sponsored study found that most patients with deep vein thrombosis should be treated with anticoagulant drugs alone, without a procedure-based intervention. However, the same study showed that a minimally-invasive catheter-director therapy, pharmacomechanical catheter-directed thrombolysis, provided greater initial pain relief and could prevent disability in DVT patients. The research was presented today […]
Elixir Medical’s DeSyne stent tops Medtronic’s Endeavor after 5 years
Elixir Medical‘s novolimus-eluting stent, DeSyne, outperformed Medtronic‘s (NYSE:MDT) zotarolimus-eluting stent, Endeavor, according to long-term data from Elixir’s Excella II trial. The study enrolled 210 patients with 2 or more de novo lesions in 2 different epicardial vessels. Researchers randomly assigned patients in a 2:1 ratio to the novolimus-eluting stent or the zotarolimus-eluting stent. The device emits a low […]
Meril’s bioresorbable, drug-eluting vascular scaffold
Meril Life Sciences has developed a drug-eluting, bioresorbable vascular scaffold that the body can slowly degrade and wash away. The company’s MeRes100 is made of biodegradable polymers, PLLA and PDLLA. Sirolimus, a commonly-used immunosuppressant found in drug eluting stents, is embedded in the scaffold’s coating. The company designed the struts of the device to be just 100 μm […]
UPDATE: Medtronic wins Health Canada license for drug-coated balloon, touts Japanese data
Medtronic (NYSE:MDT) touted 1 year data from the In.Pact SFA Japan trial of its drug-coated balloon compared to plain balloon angioplasty. The company also said today it won regulatory approval in Canada for its In.Pact Admiral DCB in patients with peripheral artery disease in the upper leg. The Japan trial enrolled 100 patients, 68 of […]
Mercator MedSystems touts data for Bullfrog micro-infusion device
Mercator MedSystems touted data today from its Dance clinical trial evaluating the company’s Bullfrog micro-infusion device as a delivery system for a generic anti-inflammatory steroid, dexamethasome. Mercator’s results were presented at the International Symposium on Endovascular Therapy and Leipzig Interventional Course. The steroid was delivered to tissues around femoropopliteal arteries after patients underwent endovascular intervention […]
Medtronic touts In.Pact Admiral drug-coated balloon data
Medtronic (NYSE:MDT) touted data for its In.Pact Admiral drug-coated balloon in patients with peripheral arterial disease. The company presented outcomes from its In.Pact Global study patient cohorts in Asia and Belgium, concluding that its DCB demonstrated consistent outcomes across patient populations. More than 96% of the 114-patient study group in Asia achieved positive outcomes, which […]
BioCardia launches pivotal trial for CardiAmp heart failure treatment
BioCardia (NSDQ:BCDA) initiated its CardiAmp pivotal trial this week to evaluate its cell therapy at 40 clinical sites across the U.S. The pivotal trial is a part of the company’s efforts for premarket approval, as regulated by the FDA’s Center for Biologics Evaluation and Research division. “We are honored to be working with the leading […]
OrbusNeich launches Combo Plus stent
OrbusNeich said today that it launched the latest version of its dual-therapy stent, the Combo Plus, featuring an antibody biological coating designed to promote vessel healing in patients with complex coronary artery disease. After the stent is implanted, the biological coating captures endothelial progenitor cells and kicks off formation of an endothelial layer. A coating of bioabsorbable polymer […]