(Image from Lyra Therapeutics)
Lyra Therapeutics (NSDQ:LYRA) announced today that it entered into a strategic partnership with LianBio for its LYR-210 treatment for chronic rhinosinusitis.
Watertown, Mass.–based Lyra, which develops the LYR-210 therapeutic to be locally delivered by its proprietary XTreo platform, could receive up to $135 million in total payments through the strategic partnership and exclusive license agreement for development and commercialization in Asia with LianBio.
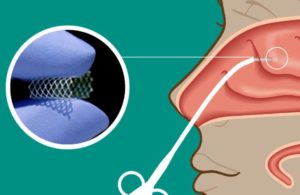
LYR-210 is designed for chronic rhinosinusitis (CRS) patients who have not had prior surgery. The XTreo delivery platform is a flexible mesh capable of taking a high payload of a drug. The platform provides a consistent release of the therapeutic over time, with Lyra touting capabilities of six months of drug delivery.
Read: How Lyra Therapeutics is advancing chronic rhinosinusitis treatment
Shanghai-based LianBio will be responsible for the clinical development and commercialization of LYR-210 in the licensed territories, which include Greater China (mainland China, Hong Kong, Taiwan and Macau), South Korea, Singapore and Thailand, according to a news release. Lyra retains all rights to the therapeutic in other geographies.
LianBio also garnered the first right to obtain development and commercial rights in the licensed territories for Lyra’s LYR-220 therapeutic for chronic rhinosinusitis patients who have undergone a prior surgery but continue to have persistent disease.
Under the agreement, Lyra will receive $12 million upfront, with up to $135 million available in future payments based on the achievement of specified development, regulatory and commercialization milestones. Lyra can also receive low double-digit royalties based on net sales of LYR-210 in the licensed territories on a region-by-region basis once commercialized.
“We are delighted to enter into this strategic alliance with LianBio to expand the global reach of LYR-210 for millions of CRS patients who need new and innovative treatment alternatives to surgery,” Lyra CEO Maria Palasis said in the release. “The LianBio team has deep expertise in drug development and is well-positioned to successfully commercialize LYR-210 in these Asian territories. The introduction of our novel, integrated ENT drug and delivery solution to the large patient populations in Asian markets supports our planned global commercialization strategy.”
LianBio CEO Yizhe Wang added that CRS patients who have failed medical management are currently left with limited and invasive options to manage their disease.
“In clinical studies conducted to date, LYR-210 has demonstrated six months of clinically meaningful symptom improvement following a single administration in patients with and without polyps,” Wang said. “With an estimated 88 million adult CRS patients in China alone, we believe LYR-210 has the potential to address a significant unmet medical need and meaningfully improve the standard of care for patients in Asia.”

