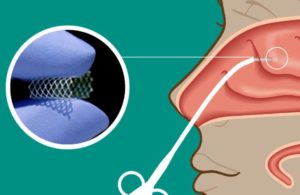
(Image from Lyra Therapeutics)
Lyra Therapeutics (NSDQ:LYRA) shares finished up on the day despite first-quarter results that missed the consensus earnings forecast.
The Watertown, Mass.-based chronic rhinosinusitis (CRS) treatment developer posted losses of -$7.8 million, or -60¢ per share for the three months ended March 31, 2021, for a -84.4% bottom-line slide. Lyra’s EPS of -60¢ came in 48¢ behind Wall Street projections.
Lyra noted that among its major recent highlights was the presentation of positive results from a Phase 2 study of its LYR-210 long-acting product for CRS, which is administered in-office to deliver a sustained release for up to six months as a non-invasive alternative to surgery for patients who have failed medical management.
Read: How Lyra Therapeutics is advancing rhinosinusitis treatment
“We were thrilled to present the full data set from our Lantern Phase 2 Study of LYR-210 at the virtual COSM event, and to share data that we believe demonstrates how effective our product candidate is at treating chronic rhinosinusitis,” Lyra president & CEO Maria Palasis said in a news release. “Additionally, we announced a separate analysis of the Lantern study that showed statistically significant improvement in a composite of three cardinal symptoms, which includes nasal blockage, nasal discharge and facial pain, that we believe could inform our design for a pivotal Phase 3 study.”
Lyra did not offer specific guidance for the full year but it stated that the company has sufficient cash to fund planned operations into 2023.
LYRA shares closed the day up 0.8% at $8.10 per share and remained unmoved after hours. MassDevice’s MedTech 100 Index — which includes stocks of the world’s largest medical device companies — finished the day down -0.8%.
