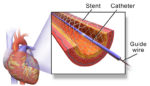
UK medtech regulators on Monday announced that manufacturers of paclitaxel-eluting balloons for use in peripheral arteries must add a warning label about the devices’ risks. A meta-analysis published in the Journal of the American Heart Association in 2018 suggested that patients treated with paclitaxel-coated balloons and stents for peripheral artery disease could be at a higher risk for late death compared […]
Biotronik
China approves Biotronik’s Orsiro drug-eluting stent
Biotronik said today that China’s National Medical Products Administration approved its Orsiro drug-eluting stent. Orsiro is a cobalt-chromium stent that elutes the drug sirolimus via the Berlin-based company’s Biolute bioabsorbable polymer coating. Biotronik said it plans to have Orsiro on the Chinese market “in the coming months.” “Orsiro will be a valuable addition to our […]
Study: No difference between Biotronik, Medtronic or Boston Scientific stents
A study comparing drug-eluting stents made by Biotronik, Medtronic (NYSE:MDT) and Boston Scientific (NYSE:BSX) found no statistically significant difference on target vessel failure or stent thrombosis after three years. The 3,514-patient Bio-Resort trial compared Biotronik’s Orsiro biodegradable sirolimus-eluting stent, Medtronic’s thin-strut zotarolimus-eluting Resolute Integrity stent and the Synergy biodegradable everolimus-eluting stent. Three-year results for some 3,393 […]
EuroPCR 2019: Elixir Medical touts DynamX
Elixir Medical touted clinical and imaging results from a study of its DynamX drug-eluting stent, which is designed to restore normal pulsatility and adaptive remodeling in blood vessels after treating a coronary lesion. DynamX, introduced in 2017 at the annual Transcatheter Cardiovascular Therapeutics meeting, is a cobalt-chromium stent with a biodegradable polymer coating that releases […]
Biotronik wins FDA nod for Orsiro DES
Biotronik said today that it won FDA approval for its for its Orsiro drug-eluting stent, touting it as the first and only ultrathin DES to outperform Abbott‘s (NYSE:ABT) Xience. The Berlin, Germany-based company said that the approval came based on data from the BIOFLOW-V pivotal trial of the device. Results from the study indicated a significantly […]
Statistical error reverses noninferiority finding in head-to-head trial of DES, bioresorbable scaffold
In a reversal, investigators for a trial comparing Biotronik‘s bioresorbable, drug-eluting Orsiro stent and Biosensors‘ BioFreedom drug-eluting stent reported this week that Biosensors’ device did not meet the criteria for noninferiority. The researchers said that while preparing the study for publication in a journal, they discovered a statistical error that ultimately changed the trial’s conclusion. […]
TCT 2018: Boston Scientific wins FDA nod for drug-eluting vascular stent
On the heels of positive study results at the 30th Transcatheter Cardiovascular Therapeutics meeting, Boston Scientific (NYSE:BSX) won FDA approval for its drug-eluting vascular stent system, Eluvia. The device, which is designed for the treatment of peripheral artery disease, uses a drug-polymer combination to deliver paclitaxel over the course of a year. “Over the past decade, we’ve […]
Biotronik touts two-year study comparing drug-eluting stents
Biotronik yesterday presented two-year data from a trial comparing Boston Scientific‘s (NYSE:BSX) Synergy biodegradable polymer everolimus-eluting stent, Biotronik’s Orsiro sirolimus-eluting stent and Medtronic‘s (NYSE:MDT) Resolute Integrity zotarolimus-eluting stent. Results from the 3,514-patient trial showed no significant difference between the various stents regarding the rate of target vessel failure after two years. But analyses of one-year data found differences between the rates of […]
Medtech stories we missed this week: April 6, 2018
From CHF Solutions’s distribution deal to Guided Therapeutics’s licensing agreement, here are seven medtech stories we missed this week but throught were still worth mentioning. 1. CHF inks Spanish distribution deal CHF Solutions announced in an April 5 press release that it has signed a distribution agreement with Dimedix Surgical. The distribution agreement will allow […]
Biotronik’s Orsiro drug-eluting stent succeeds at five-year follow-up
Biotronik touted long-term safety and efficacy data for its Orsiro drug-eluting stent yesterday at this year’s Transcatheter Cardiovascular Therapeutics meeting. Data from the Bioflow-II trial and the Bioflow-III registry demonstrated that Biotroniks’s device boasts strong safety and clinical performance at five years, the company reported. In the 268-patient group treated with Orsiro, 60-month data from […]