Bayer (ETR:BAYN) led a $40 million Series B round for glucose monitor maker One Drop, licensed its platform for treating conditions beyond diabetes and put its pharma president on the One Drop board. New York City-based One Drop developed a compact blood glucose monitor, pairing it with an app designed to provide behavioral cues and one-on-one coaching. […]
Dexcom claims win in patent war with AgaMatrix unit WaveForm
Dexcom Inc. (NSDQ:DXCM) last week claimed a win along one front of its patent war with AgaMatrix subsidiary WaveForm Technologies, when an Oregon federal court dismissed a lawsuit alleging that its continuous glucose monitors infringe WaveForm patents. Wilsonville, Ore.-based WaveForm sued Dexcom in March 2016, alleging infringement of a trio of patents covering a compound […]
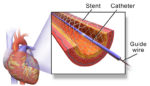
Study: No difference between Biotronik, Medtronic or Boston Scientific stents
A study comparing drug-eluting stents made by Biotronik, Medtronic (NYSE:MDT) and Boston Scientific (NYSE:BSX) found no statistically significant difference on target vessel failure or stent thrombosis after three years. The 3,514-patient Bio-Resort trial compared Biotronik’s Orsiro biodegradable sirolimus-eluting stent, Medtronic’s thin-strut zotarolimus-eluting Resolute Integrity stent and the Synergy biodegradable everolimus-eluting stent. Three-year results for some 3,393 […]
Veramorph lands spot in Delaware accelerator
Veramorph Materials landed a spot in a Delaware business accelerator backed by DuPont to develop its hydrogel-based oral drug delivery platform. Veramorph and MCET Technologies (which is developing wearable sensor technology) won their spots in the Delaware Innovation Space via the DuPont FastPass competition. The companies get a year of free access to the accelerator, […]
Pfizer to absorb Mylan in off-patent drug spinout
Pfizer (NYSE:PFE) plans to absorb Mylan (NSDQ:MYL) as part of the spinout of its Upjohn off-patent drugs business in an all-stock deal. The deal, structured to avoid taxes on any gains from the spinout, would see each Mylan share converted into a share of the as-yet-unnamed new company. Pfizer stockholders would then own 57% of […]
FDA approves delivery system for Intersect ENT’s Propel implant
Intersect ENT (NSDQ:XENT) said today that it won FDA approval for a new delivery system for its Propel Mini steroid-eluting sinus implant. The Menlo Park, Calif.-based company said the straight delivery system is designed to place the mometasone furoate-eluting Propel Mini in the ethmoid sinus behind the nasal bridge. The SDS is slated to hit […]
Livongo share prices double after upsized $356m IPO
Investors added nearly 50% to the share price for Livongo Health (NSDQ:LVGO) today after it raised $355 million with its initial public offering after a last-minute upsizing. The Mountain View, Calif.-based company, which developed a digital health platform to manage chronic diseases, said earlier this week that it planned to float 10.7 million shares at $24 […]
Eli Lilly, Aptar win FDA approval for nasal glucagon powder
Eli Lilly (NYSE:LLY) and AptarGroup (NYSE:ATR) today won FDA approval for the first injection-free glucagon for treating severe low blood sugar in diabetes patients. The federal safety watchdog’s approval, of Lilly’s Baqsimi nasal powder and the delivery device developed by Aptar for treating severe hypoglycemia, was based on two studies comparing a single dose of Baqsimi […]
Livongo boosts IPO to $268m midpoint
Livongo Health yesterday boosted the range on its pending initial public offering, saying the flotation would now fetch nearly $268 million at the midpoint. The Mountain View, Calif.-based company developed a digital health platform to manage chronic diseases, selecting diabetes as its first target. After a series of upsizing announcements, Livongo yesterday said it plans […]
Medtronic bids for expanded FDA indication for Guardian Sensor 3
Medtronic (NYSE:MDT) said it asked the FDA for an expanded indication for its Guardian Sensor 3 device that would open the glucose sensor up for Medicare reimbursement. The Fridley, Minn.-based medtech titan said it filed for pre-market approval of non-adjunctive labeling for Guardian Sensor 3 for use with its MiniMed 670G insulin management system. If […]