PAVmed (Nasdaq:PAVM) has successfully completed the first human implants of its PortIO intraosseous infusion system, the company said today. New York-based PAVmed designed its PortIO system with an implantable intraosseous vascular access device and insertion kit. Instead of a catheter located in a vein, the system has a short extension from the device inserted by […]
Implants
Second Sight Medical, Nano Precision Medical merging to create therapeutic implant company
Second Sight Medical Products (NSDQ:EYES) and Nano Precision Medical agreed to a merger deal that will focus on drug-device medical implants. The two companies entered into a definitive agreement under which privately held Nano Precision Medical (NPM) will merge with a wholly-owned subsidiary of Second Sight in an all-stock transaction, with NPM the surviving company […]
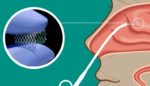
Lyra Therapuetics initiates Phase 3 trial for chronic rhinosinusitis treatment
Lyra Therapeutics (NSDQ:LYRA) announced today that it initiated the Phase 3 Enlighten I trial for its LYR-210 treatment. Watertown, Massachusetts-based Lyra’s Phase 3 Enlighten I clinical trial will evaluate LYR-210, delivered through Lyra’s proprietary XTreo drug-eluting implant, in adult, surgically naïve chronic rhinosinusitis (CRS) patients. The company designed LYR-210 for delivery in a brief, non-invasive, […]
Ocular Therapeutix touts positive trial results of ophthalmic insert for dry eye disease
Ocular Therapeutix (NSDQ:OCUL) announced today that it reported positive results from a Phase 2 trial of its dry eye disease treatment. Bedford, Massachusetts-based Ocular Therapeutix’s U.S.-based Phase 2 clinical trial evaluated OTX-DED (dexamethasone intracanalicular ophthalmic insert) for the short-term treatment of dry eye disease. The randomized, double-masked, vehicle-controlled, multi-center trial evaluated two different formulations of […]
Ocular Therapeutix claims win as court invalidates drug delivery patent from Mati
Ocular Therapeutix (NSDQ:OCUL) announced today that an appeals court invalidated a drug delivery system patent held by Mati Therapeutics. The U.S. Court of Appeals for the Federal Circuit in Washington, D.C., invalidated the patent related to a drug delivery system containing dexamethasone, affirming the judgment made in June 2020 by the Patent Trial and Appeal […]
FDA approves Genentech’s Susvimo drug-eluting eye implant
Genentech announced today that it received FDA approval for its Susvimo injection for treating macular degeneration (AMD). South San Francisco-based Genentech, a member of the Roche Group, designed its Susvimo ranibizumab injection (100 mg/mL) for intravitreal use via ocular implant for treating people with wet, or neovascular, age-related macular degeneration (AMD) who have previously responded […]
Ivantis to pay $60M in patent litigation settlement with Glaukos
Glaukos (NYSE:GKOS) announced today that it entered into a settlement with Ivantis to terminate a three-year-old patent infringement lawsuit. San Clemente, California-based Glaukos’ patent infringement lawsuit, initiated on April 14, 2018, in the U.S. District Court for the Central District of California, Southern Division, concerned Ivantis’ Hydrus Microstent for reducing eye pressure in glaucoma patients. […]
Lyra Therapeutics appoints new CFO
Lyra Therapeutics (NSDQ:LYRA) announced today that it appointed Jason Cavalier as its new chief financial officer, effective today. Cavalier succeeds the company’s current CFO, Don Elsey, who is retiring while he remains expected to serve in an advisory role to assist with the transition. Watertown, Massachusetts-based Lyra — which develops the XTreo platform designed to […]
Lyra touts non-human trial of XTreo drug-eluting implant
Lyra Therapeutics (NSDQ:LYRA) is touting preclinical data for its XTreo drug-eluting implant for dosing in local sinus tissues. Watertown, Massachusetts-based Lyra’s preclinical results were published — “Drug Release and Pharmacokinetic Evaluation of Novel Implantable Mometasone Furoate Matrices in Rabbit Maxillary Sinuses” — were published online in the peer-review journal, American Journal of Rhinology & Allergy. The […]
Glaukos submits supplemental PMA application for iStent Infinite
Glaukos (NYSE:GKOS) announced today that it submitted a supplemental premarket approval application to the FDA for its iStent Infinite system. San Clemente, California–based Glaukos designed the iStent Infinite trabecular micro-bypass system for use in a standalone procedure to reduce elevated intraocular pressure (IOP) in patients with open-angle glaucoma uncontrolled by prior surgery or medical therapy. […]