Glucotrack (Nasdaq:GCTK) announced that it appointed Drinda Benjamin as its new vice president of marketing. Rutherford, New Jersey-based Glucotrack develops an implantable continuous glucose monitor (CGM). It designed its CGM for patients with type 1 and type 2 insulin-dependent diabetes. A recent feasibility study demonstrated reliable glucose measurements for two years post-implant for the device. […]
Implants
BioCorRx submits implantable opioid use disorder treatment to FDA for expanded access
BioCorRx announced today that it submitted to the FDA an expanded access program application for its BICX104 implantable naltrexone pellet. BICX104, a biodegradable implantable pellet, treats opioid use disorder (OUD). The expanded access program would enable the treatment to reach eligible patients before full FDA approval. The FDA program provides access to products before approval […]
This biodegradable brain implant delivers cancer-treating drugs
Researchers say they developed a biodegradable brain implant capable of helping to deliver chemotherapy drugs directly to tumors. Medscape News reported that the research marks another step toward using ultrasound to combat cancer. According to the team, led by Thanh Nguyen, these drugs can penetrate the blood-brain barrier to reach these brain tumors. Nguyen serves […]
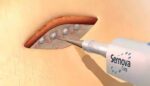
Sernova reports positive interim data for Cell Pouch System
Sernova today announced positive interim data from the clinical trial of its Cell Pouch System in patients with type 1 diabetes. London, Ontario-based Sernova designed its proprietary Cell Pouch System as an implantable and scalable medical device. It forms a natural environment in the body for the long-term survival and function of therapeutic cells. Those […]
UnitedHealthcare to cover Senseonics Eversense E3 CGM
Senseonics (NYSE:SENS) announced that UnitedHealthcare intends to begin providing coverage for its Eversense E3 CGM. Coverage of the continuous glucose monitor for people with type 1 and insulin-requiring type 2 diabetes goes into effect on July 1, 2023. UnitedHealthcare, the largest health insurance company in the U.S., covers more than 45 million lives. Its offerings […]
Ascensia expands payment program for Senseonics Eversense E3 CGM
Ascensia Diabetes Care announced today that it expanded the Eversense PASS program for the Senseonics (NYSE:SENS) Eversense E3 CGM. Eversense PASS, a payment assistance and simple savings program, aims to enhance affordability and access to Eversense E3. Ascensia serves as the global commercial partner for Senseonics. The Senseonics continuous glucose monitor (CGM), a 180-day implantable […]
Senseonics announces first pediatric 365-day CGM insertion
Senseonics (NYSE:SENS) announced that the first pediatric study participant received an Eversense 365-day CGM insertion. The insertion took place as part of the company’s ENHANCE clinical trial at the AMCR Institute. AMCR, a clinical research center, focuses on pre-diabetes, type 1, type 2 diabetes and obesity. The study operates under the direction of Dr. Timothy […]
Researchers use tiny drug delivery implant to treat tumors
Researchers at Houston Methodist Research Institute developed a tiny drug delivery device for addressing difficult-to-treat cancers. The researchers published their work in a paper in Advanced Science. They used an implantable nanofluidic device they invented to deliver CD40 monoclonal antibodies (mAB). The team calls the device a nanofluidic drug-eluting seed (NDES). With the device — […]
These ear tubes with liquid infused materials may improve ear infection treatment outcomes
A research collaboration in Boston may have uncovered a design overhaul to improve outcomes for ear tubes known as tympanostomy tubes (TTs). These TTs create an opening between the ear canal and middle ear. Their aim is to ventilate the middle ear, provide a route for fluid to drain out and allow antibiotic drops to […]
Celanese to provide drug delivery platform for Glaukos glaucoma treatment
Celanese (NYSE:CE) announced today that it entered into an agreement with Glaukos (NYSE:GKOS) over drug delivery for glaucoma treatments. Dallas-based Celanese agreed to supply its VitalDose drug delivery platform for use as a component in Gluakos’ iDose TR. The iDose TR serves as a micro-invasive intraocular implant for lowering intraocular pressure. It treats patients with […]